Solved 8. BeCl2 Name Lewis Structure 3D Drawing Molecular
Becl2 or Beryllium Chloride is an inorganic compound which is hygroscopic in nature and soluble in only polar solvents. It can be either white color solid or colorless in nature. The properties of BeCl2 is quite similar to aluminum chloride due to diagonal relationship. How to draw Becl2 lewis structure?

BeCl2 Lewis Structure & VSEPR Geometry YouTube
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
[Solved] 10. (5 pts.) BeCl2 LEWIS STRUCTURE WITH ANY RESONANCE
Lewis structure of BeCl2 contains two single bonds between the Beryllium (Be) atom and each Chlorine (Cl) atom. The Beryllium atom (Be) is at the center and it is surrounded by 2 Chlorine atoms (Cl). The Beryllium does not have lone pairs while both the Chlorine atoms have 3 lone pairs.

Becl2 Electron Pair Geometry
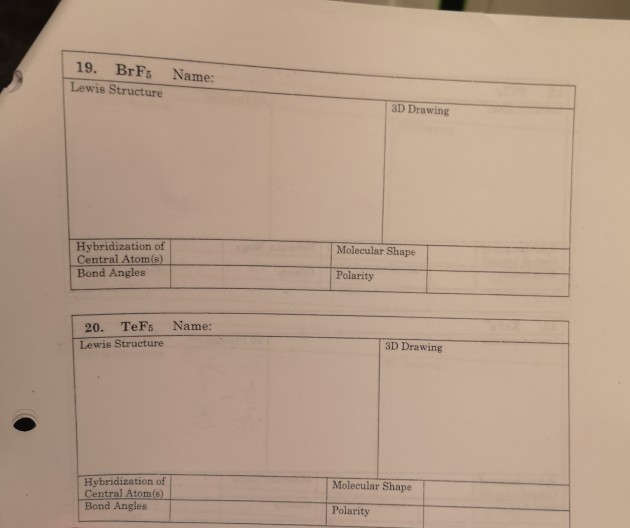
The beryllium atom in a gaseous BeCl2 molecule is an example of a central atom with no lone pairs of electrons in a linear arrangement of three atoms.. (90°). Sulfur is in the same group as oxygen, and H 2 S has a similar Lewis structure. However, it has a much smaller bond angle (92.1°), which indicates much less hybridization on sulfur.

Is BeCl2 Polar or Nonpolar? Techiescientist
A step-by-step explanation of how to draw the BeCl2 Lewis Dot Structure (Beryllium chloride).For the BeCl2 structure use the periodic table to find the total.

How to draw BeCl2 Lewis Structure? Science Education and Tutorials
BeCl2 Lewis Structure - How to Draw the Lewis Structure for BeCl2 Wayne Breslyn 721K subscribers Join Subscribe Subscribed 1.1K 161K views 10 years ago A step-by-step explanation of how to.
BeCl_2 and TeCl_2 are both covalent molecules, yet BeCl_2 is linear
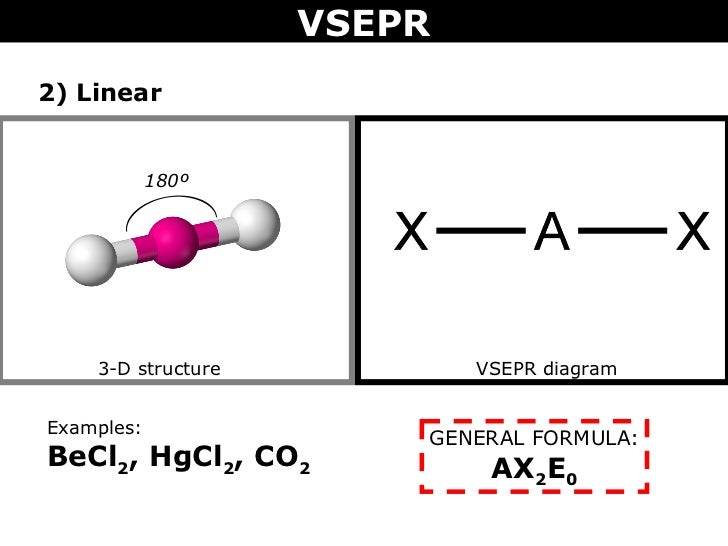
The BeCl 2 Lewis structure is similar to BeF 2 since F is in Group 7 and has 7 valence electrons. Beryllium (Be) doesn't need 8 valence electrons to have an octet (Be often only needs 4). If you're not sure you have the best Lewis structure for BeCl 2 you can calculate the formal charges. You'll find the Be in BeCl 2 only has 4 valence electrons.

Question 10.19 Draw the structure of (i) BeCl2 (vapour) (ii) BeCl2
Beryllium chloride has a significant ionic contribution to its electronic structure. A quick calculation, DF-BP86/def2-SVP, reveals that the charge of beryllium is q(Be) = 0.8 q ( B e) = 0.8 and of chlorine q(Cl) = −0.4 q ( C l) = − 0.4, based on natural bond orbital analysis. The Lewis structures best describing this are depicted below.
25 Bohr Diagram For Beryllium Wiring Database 2020
According to the VSEPR theory, BeCl2 possesses a linear molecular geometry and a BeCl2-like electron geometry. Because the center atom, beryllium, has two Be-Cl bonds with the two chlorine atoms surrounding it. The Cl-Be-Cl bond generates a 180-degree angle in the linear geometry. The BeCl2 molecule has a linear shape because it contains two.
Fcl2 Lewis Structure What is the molecular shape of BeCl2? YouTube
BeCl2 Lewis structure contains beryllium atom in central position whereas both chlorine atom on either side of it. There is no lone pair present on the central atom but 3 lone pairs present on each outer atom in the lewis dot structure of BeCl2.
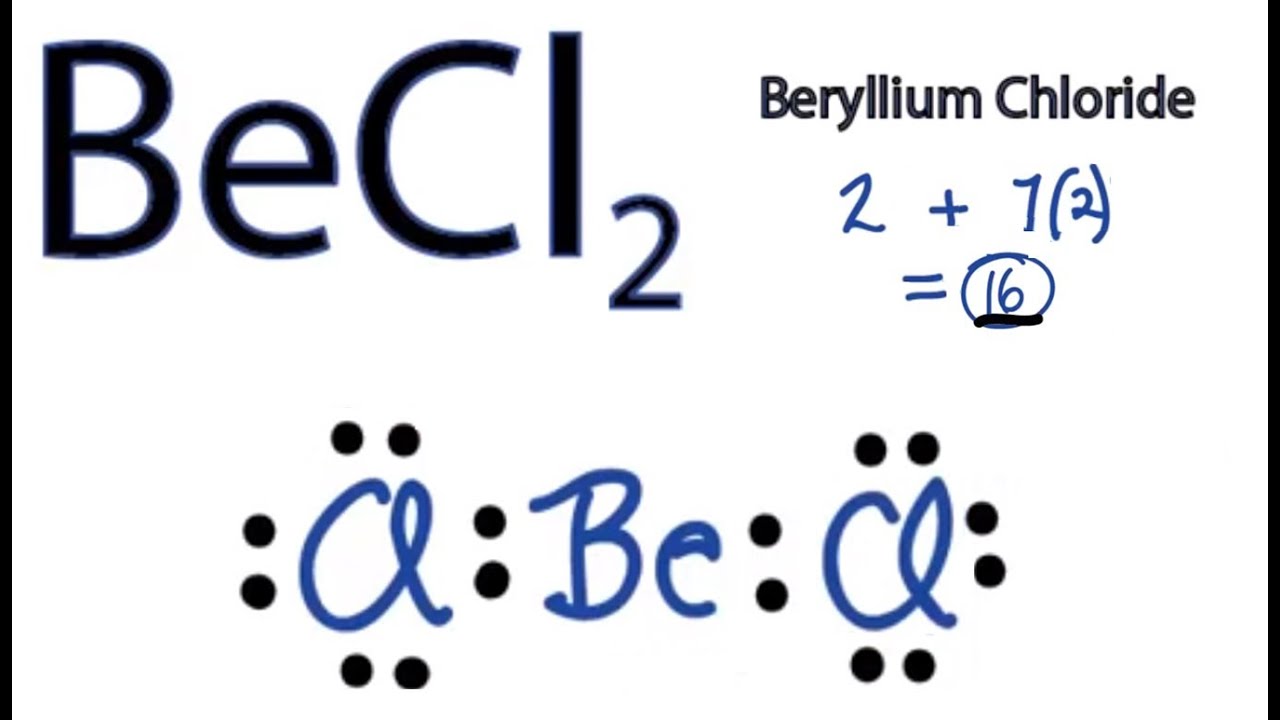
BeCl2 Lewis Structure How to Draw the Lewis Structure for BeCl2 YouTube
Electron Deficient Species. Good examples of the first type of exception are provided by BeCl 2 and BCl 3.Beryllium dichloride, BeCl 2, is a covalent rather than an ionic substance.Solid BeCl 2 has a relatively Complex structure at room temperature, but when it is heated to 750°C, a vapor which consists of separate BeCl 2 molecules is obtained. Since Cl atoms do not readily form multiple.

BeCl2 Molecular Geometry Science Education and Tutorials
BeCl2 is a molecular (covalent) compound in the gas phase. It is a linear molecule and beryllium is an exception to the octet rule.In the solid phase, you ca.
/Lewis-dot-58f78f405f9b581d5938e617.jpg)
Exceptions to the Octet Rule
Here's how you can easily draw the BeCl 2 Lewis structure step by step: #1 Draw a rough skeleton structure. #2 Mention lone pairs on the atoms. #3 If needed, mention formal charges on the atoms. Now, let's take a closer look at each step mentioned above.

What is the structure of becl2 molecule in gaseous and solid state
BeCl2 lewis structure has a Beryllium atom (Be) at the center which is surrounded by two Chlorine atoms (Cl). There are 2 single bonds between the Beryllium atom (Be) and each Chlorine atom (Cl). There are 3 lone pairs on both the Chlorine atoms (Cl).

BeCl2 Lewis structure, Molecular geometry, Hybridization, Bond angle
Subscribed 24K views 3 years ago An explanation of the molecular geometry for the BeCl2 (Beryllium chloride) including a description of the BeCl2 bond angles. The electron geometry for the.

BeCl2 Lewis Structure (Beryllium Chloride) YouTube
The Lewis structure of BeCl2 presents that Be has two valence electrons, and Cl has seven valence electrons. Steps to draw the Lewis Structure of BeCl2 Follow the below-mentioned steps to draw the Lewis structure of BeCl2 Step 1: The number of valence electrons in beryllium chloride molecule has to be counted first.